Menu
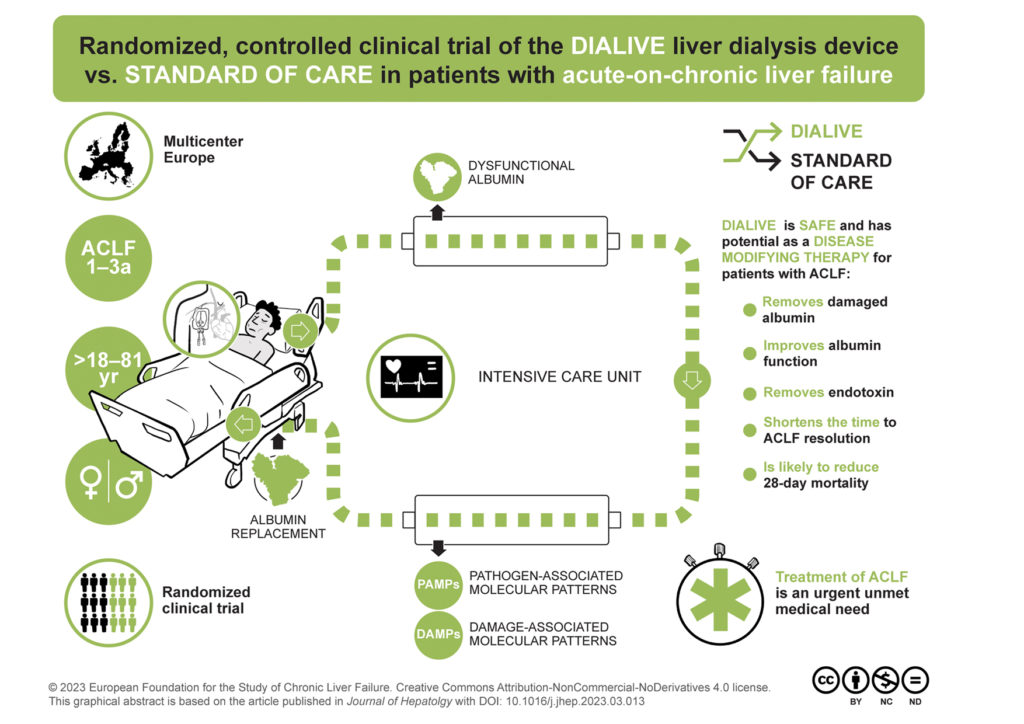
As opposed to other liver assist devices, DIALIVE was designed to specifically address the physiopathological derangements that lead to ACLF and, thus, replace dysfunctional albumin, and remove excess of damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs), which mediate an excessive inflammatory response.
Previous pre-clinical studies showed increased survival in animal models of liver failure demonstrating the promising potential of DIALIVE. Within the framework of the EU-funded ALIVER project, researchers carried out the first-in-human randomized clinical trial to assess safety and effectiveness of DIALIVE treatment compared with standard of care. Researchers also looked at the clinical benefit of DIALIVE, this means, the impact of treatment on patients outcomes including resolution of ACLF and prognosis improvement.
The study, led by Banwari Agarwal, consultant in intensive care medicine at Royal Free Hospital, UK, involved multiple centers across Europe and recruited 32 patients with underlying alcohol-related hepatitis and ACLF. Treatment last for up to 5 days and serious adverse effects were monitored. Compared with standard of care, this study confirmed that DIALIVE is safe and has potential as a disease-modifying therapy for patients with ACLF. Patients showed significant improvement in albumin function, reduction of circulating endotoxin, reduction of systemic inflammation markers, reduction of cellular components released from damaged or dying cells, restoration of endothelial function, and reduction of markers associated with brain and renal dysfuntion. The most striking finding was that a proportion of patients treated with DIALIVE resolved ACLF faster than those receiving standard of care, and patients that resolved remained free of ACLF for 28 days after only 3 days of treatment.
“This is the first treatment that has shown such a rapid clinical effect in critically ill patients with ACLF”, said co-senior author Rajiv Jalan, Principal Investigator at EF CLIF, Spain, and at University College London, UK.
DIALIVE did not reduce mortality at 28 days in this small study and larger clinical trials are needed to confirm the efficacy of this liver dialysis device.
“DIALIVE is a promising therapeutic option for ACLF. However, larger studies are required to confirm this hypothesis and clarify two main clinical issues: 1) which patients benefit most from the intervention (target population), and 2) what is the most appropriate time for the therapy”, said co-senior author Javier Fernández, Principal Investigator at EF CLIF and Head of the Liver Intensive Care Unit at Hospital Clínic de Barcelona, Spain.
“If proven effective in a larger clinical trial, it is conceivable that DIALIVE may help in the appropriate selection and management of patients diagnosed with ACLF and with potential indication of liver transplantation. Furthermore, the potential improvement in the natural course of ACLF may also impact in the length of hospital stay also shortening intensive care unit requirements”, said co-first author Rafael Bañares, Head of the Gastroenterology and Hepatology Department at Hospital Universitario Gregorio Marañón, Spain. “Considering that the pathophysiological effects of DIALIVE and the beneficial clinical signs are observed after a limited number of sessions it would be important to evaluate wether an increase in the duration of therapy could impact in relevant clinical outcomes such as survival, he concluded.
Other authors on the study are Banwari Agarwal, Faouzi Saliba, Maria Pilar Ballester, Dana Rodica Tomescu, Daniel Martin, Vanessa Stadlbauer, Gavin Wright, Mohammed Sheikh, Carrie Morgan, Carlos Alzola, Phillip Lavin, Daniel Green, Rahul Kumar, Sophie Caroline Sacleux, Gernot Schilcher, Sebastian Koball, Andrada Tudor, Jaak Minten, Gema Domenech, Juan Jose Aragones, Karl Oettl, Margret Paar, Katja Waterstradt, Stefanie M. Bode-Boger, Luis Ibáñez-Samaniego, Amir Gander, Carolina Ramos, Alexandru Chivu, Jan Stange, Georg Lamprecht, Moises Sanchez, Rajeshwar P. Mookerjee, Andrew Davenport, Nathan Davies, Marco Pavesi, Fausto Andreola, Agustin Albillos, Jeremy Cordingley, Hartmut Schmidt, Juan Antonio Carbonell-Asins, Vicente Arroyo, and Steffen Mitzner.

This study received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement no. 733057.
Agarwal, B.; Bañares Cañizares, R.; Saliba, F.; Ballester, M.P.; Tomescu, D.R.; Martin, D.; Stadlbauer, V.; Wright, G.; Sheikh, M.; Morgan, C.; Alzola, C.; Lavin, P.; Green, D.; Kumar, R.; Sacleux, S.C.; Schilcher, G.; Koball, S.; Tudor, A.; Minten, J.; Domenech, G.; Aragones, J.J.; Oettl, J.; Paar, M.; Waterstradt, K.; Bode-Boger, S.M.; Ibáñez-Samaniego, L.; Gander, A.; Ramos, C.; Chivu, A.; Stange, J.; Lamprecht, G.; Sanchez, M.; Mookerjee, R.P.; Davenport, A.; Davies, N.; Pavesi, M.; Andreola, F.; Albillos, A.; Cordingley, J.; Schmidt, H.; Carbonell-Asins, J.A.; Arroyo, V.; Fernandez, J.; Mitzner, S.; Jalan, R. Randomized, controlled clinical trial of the DIALIVE liver dialysis device versus standard of care in patients with acute-on-chronic liver failure. J. Hepatol. 2023, 79(1). DOI: 10.1016/j.jhep.2023.03.013
European Foundation for the Study of
Chronic Liver Failure
Avinguda Diagonal 477, 11th floor
08036 Barcelona, Spain
Tel: +34 93 227 14 00
Email: Send us an email
© 2025 European Foundation for the Study of Chronic Liver Failure

| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |